
In Chemistry covalent bond is a link between two ions or atoms in which a pair of electrons is shared between them. The covalent bond also termed as a molecular bond. The covalent bond formed between two nonmetal atoms whose electronegativity values are equal or relatively similar. In 1919, the term “covalence” was coined by Irving Langmuir, defining the number of electron pairs exchanged by the adjacent atoms. However, in 1939, the name “covalent bond” was first described.
The electron pairs involved in a covalent bond are called shared pairs or bonding pairs. Usually exchanging bonding pairs causes each atom to achieve a stable state as seen in noble gas that will have a stable electron outer shell. The valence shell of some atoms have no stable configuration, so all the atoms having less than eight electrons in their valence shell except noble gases.
The concept of obtaining a maximum of eight electrons in the valence shell of atoms is called the octet rule.
Types of Covalent Bond
There are different types of covalent bonds depending on the sharing of electrons between the participating atoms such as single covalent bond, double covalent bond, triple covalent bond and polar bonds.
1. Single Covalent Bond
Two participating atoms form a single covalent bond by sharing only one pair of electrons between them.
Example:
HCl molecule, in which hydrogen molecule has one electron in the outermost shell and chlorine atom has seven electrons in their valence shell. A single covalent bond is formed between H and Cl atoms by sharing one electron each.
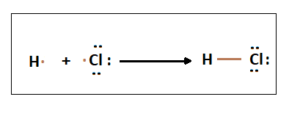
2. Double Covalent Bond
Two participating atoms form two covalent bonds by sharing two pairs of electrons between them and they are represented by “=” (two dashes). Double bonds are less stable but stronger than a single bond.
Example:
A CO2 molecule has two oxygen atoms with four valence electrons and one carbon atom with six valence electrons. A carbon atom to achieve stable state (octet), it shares a total of four valence electrons with two oxygen atoms to form double bonds.
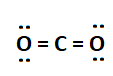
3. Triple Covalent Bond
Two participating atoms form three covalent bonds by sharing three pairs of electrons between them and they are represented by“≡” (three dashes) and the bond is less stable than a double bond.
Example:
Formation of nitrogen molecules, each atom has five electrons in the outermost shell. By sharing three electrons from each atom form three covalent bonds.
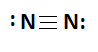
4. Polar Covalent Bond
During the formation of covalent bonds, two participating atoms will have differences in electronegativity value (zero to two). Due to this reason, there is an unequal sharing of electrons between the atoms. These types of bonds are called polar covalent bonds.
Example of polar covalent bonds is water (H2O). The electronegativity of oxygen is 3.44 and hydrogen is 2.20.
Another example of a polar covalent bond is HF. Fluorine is more electronegative therefore electrons are more towards Fluorine atom.
5. Nonpolar covalent Bond
During the formation of covalent bonds, two participating atoms will have equal electronegativity value (zero) and there is an equal sharing of electrons between the participating atoms. These types of bonds are called nonpolar covalent bonds or pure covalent bonds.
An Example of nonpolar covalent bonds is H2 gas (both are identical atoms). According to chemists, the electronegativity difference between the participation atoms is less than 0.4 (e.g. carbon dioxide, methane) are considered as a nonpolar covalent bond.

